Clinical Study #1
Background & Aim
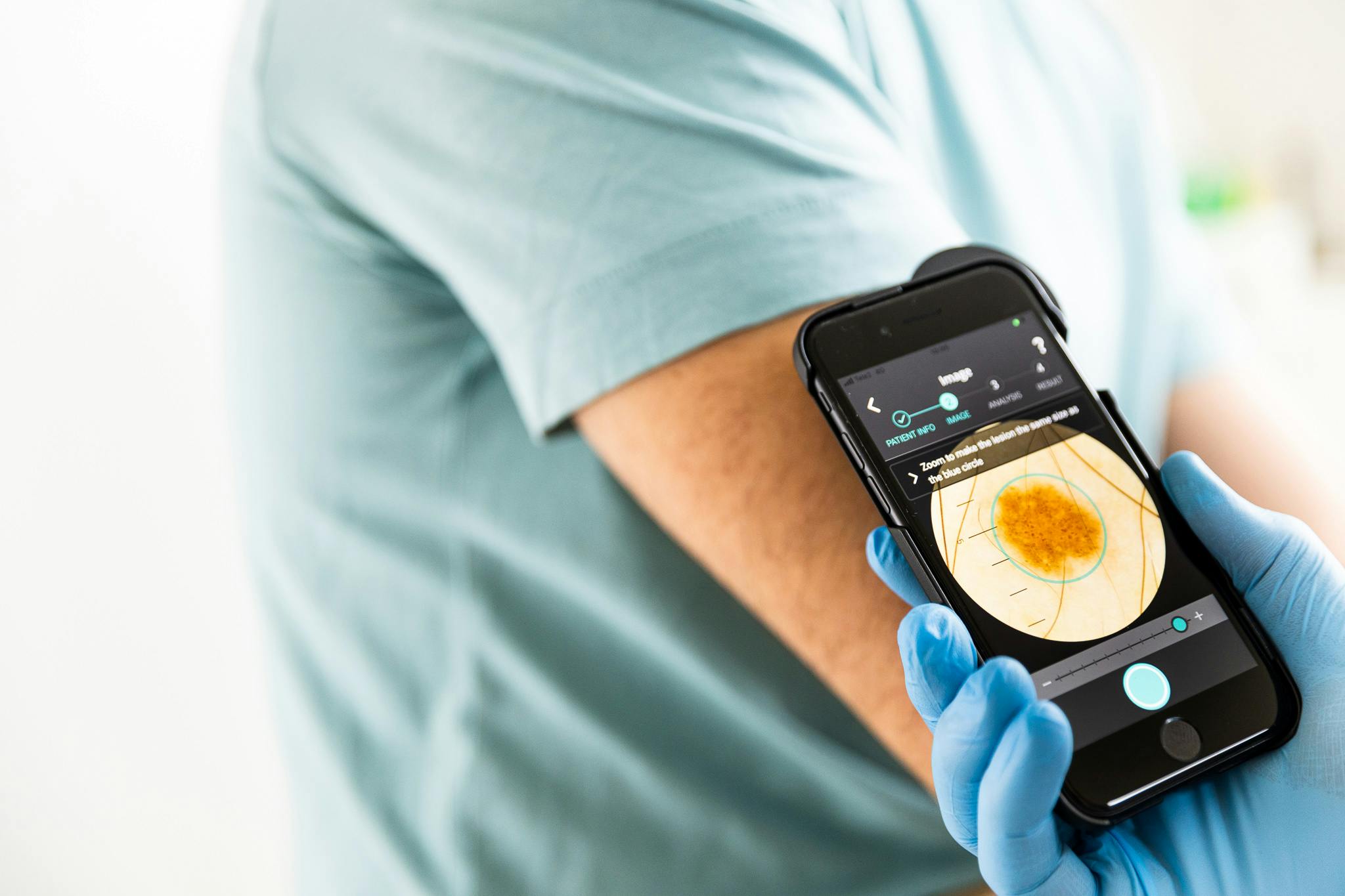
The study's primary objective was to determine the diagnostic precision of the AI-based medical device Dermalyser by answering at which level Dermalyser can identify melanomas among cutaneous lesions assessed in clinical use due to any degree of malignancy suspicion.
The primary endpoint was measured by testing if Dermalyser gives correct results as compared with the final diagnosis of the lesion analysis (the final classification by histopathology (PAD) and/or Dermatologist assessment) in at least a certain proportion (π) of the analyses.
A secondary objective was to evaluate the usability and applicability in the clinical practice of Dermalyser by medical professionals and to gain an increased knowledge and understanding of how digital tools enhanced by AI can assist physicians with the proper support for an earlier diagnosis of melanoma. Towards this goal, users were asked to rate Dermalyser via a series of questions, including a System Usability Scale.

Study Details
Number of Subjects
241 subjects from 37 primary care centres in Sweden.
Diagnosis and main eligibility criteria
Patients ≥18 years.
Patients attending a primary care facility with at least 1 suspicious skin lesion where MM cannot be ruled out.
Willingness and ability to provide informed consent.
Summary of Results
The study demonstrates an AUC of 0.96, and after threshold calibration, the result from the clinical investigation demonstrates an AI performance of 95% sensitivity and 85.5% specificity.
To read further details of the study, visit:
https://clinicaltrials.gov/
For any questions about the study or to learn more about Dermalyser, please contact us.